Acidified Potassium Manganate Vii Formula
The alkene monomer has the general formula. All alkenes will react with free radical initiators to form polymers by a free radical addition reaction.
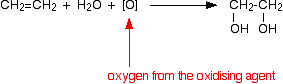
Oxidation Of Alkenes With Potassium Manganate Chemistry Libretexts
D AND f BLOCK ELEMENTS TINTO JOHNS M.
. D and f block elements 1. The situation with acidified potassium manganateVII solution is even worse because it has a tendency to break carbon-carbon bonds. It is a reasonably strong oxidising agent without being so powerful that it takes the whole of the organic molecule to pieces.
MELTING POINT AND BOILING POINT High MP and BP - Due to strong metallic bond and the presence of half filled d-orbitals Involvement of greater number of electrons from n-1d in addition to the ns electrons in the inter atomic metallic bonding. You could use alkaline potassium manganateVII solution if for example all you had to do was to find out whether a. Solution Y is colourless.
1 ii Give one difference in the atomic. Eg ethene reacts with acidified potassium manganate VIIaq to give ethan-12-diol 3 Addition polymerisation. 20 Solution X is colourless.
No change is seen. 4 UCLES 2021 507022MJ21 2 Oxygen sulfur selenium tellurium and polonium are in Group VI. Where R is any group of atoms eg RCH 3 for propene.
Academiaedu is a platform for academics to share research papers. Potassium dichromateVI solution acidified with dilute sulphuric acid is commonly used as an oxidising agent in organic chemistry. Monomer - a single unit eg an alkene.
A few drops of aqueous potassium iodide solution are added to a sample of X. NCERT Solutions for Class 12 Chemistry Chapter 8 Free PDF Download. Academiaedu is a platform for academics to share research papers.
NCERT Solutions for Class 12 Chemistry Chapter 8 The d and f Block Elements is a powerful study material that has answers to textbook exercises and important questions from the previous year and sample papers. The colour of the potassium manganateVII disappears. It reacts destructively with a large number of organic compounds and is rarely used in organic chemistry.
1 c Two isotopes of polonium are shown. State and describe the establishment of ethanoic acid by the oxidation of ethanol by fermentation and through acidified potassium manganateVII Describe and explain ethanoic acid as a typical weak acid. Assess and evaluate the reaction of a carboxylic acid with an alcohol in the presence of a catalyst to give an ester.
A State the percentage composition by volume of oxygen in dry air. Oxidise secondary alcohols to ketones. Potassium manganateVII solution has some tendency to do that It is used to.
20 8 9 4Po 22 1 8 0 4Po i Explain why both isotopes have the same chemical properties. 1 b State one large-scale use for oxygen. A few drops of aqueous acidified potassium manganateVII solution are added to a sample of Y.
These NCERT Solutions for Class 12 Chemistry are prepared based on the latest CBSE.
How Oxidation By Potassium Manganate Vii Kmno4 Take Place Tutorke
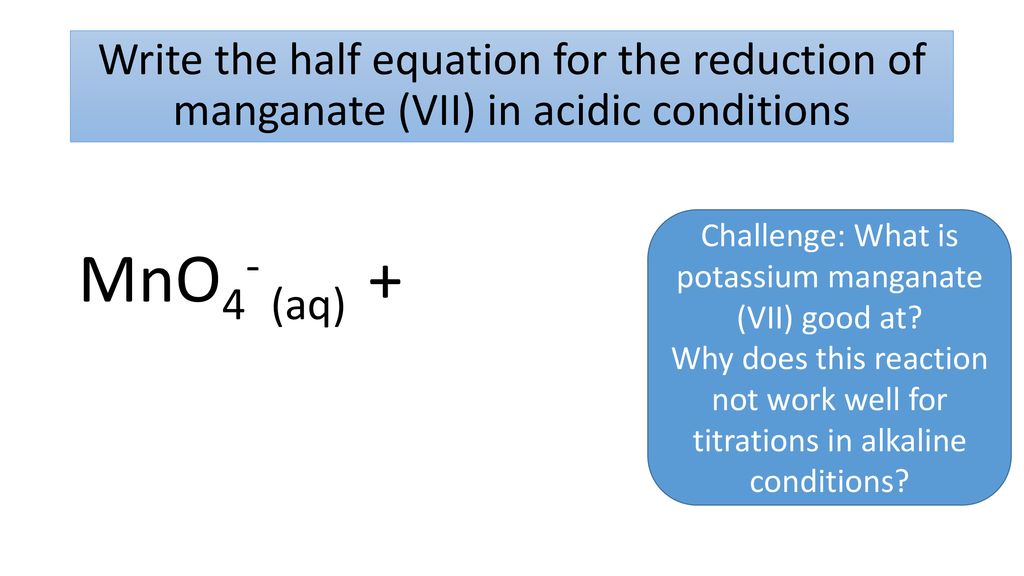
Challenge What Is Potassium Manganate Vii Good At Ppt Download

Challenge What Is Potassium Manganate Vii Good At Ppt Download
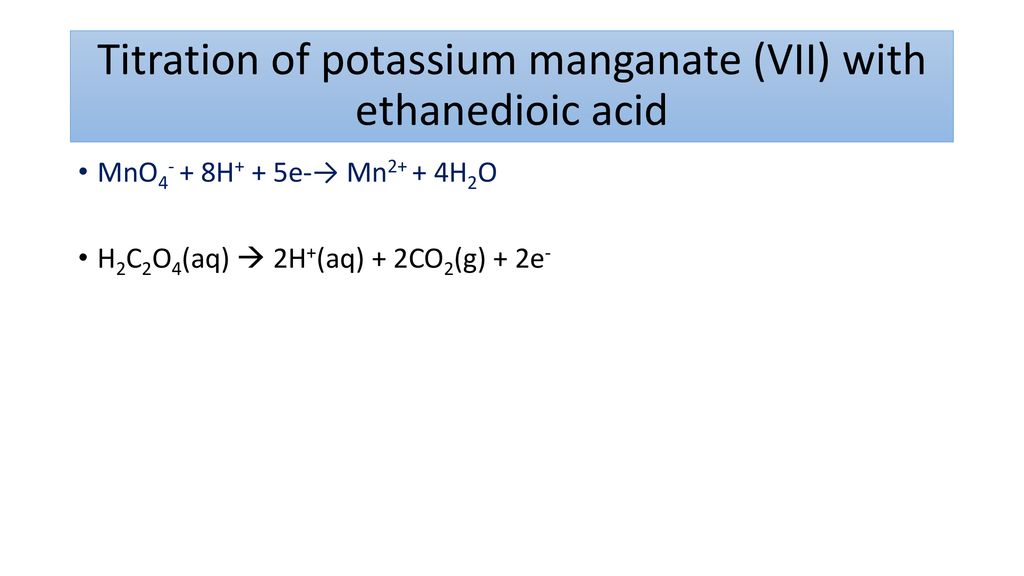
Challenge What Is Potassium Manganate Vii Good At Ppt Download

Comments
Post a Comment